ADCs are a promising class of cancer drugs designed to deliver potent cell-killing agents directly to tumors. This targeted approach aims to minimize side effects often experienced with traditional chemotherapy. Here's a breakdown of how ADCs work on a molecular level.
The Building Blocks of an ADC:
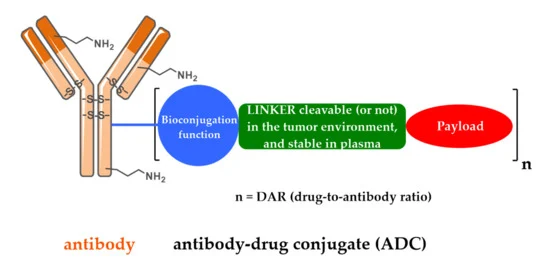
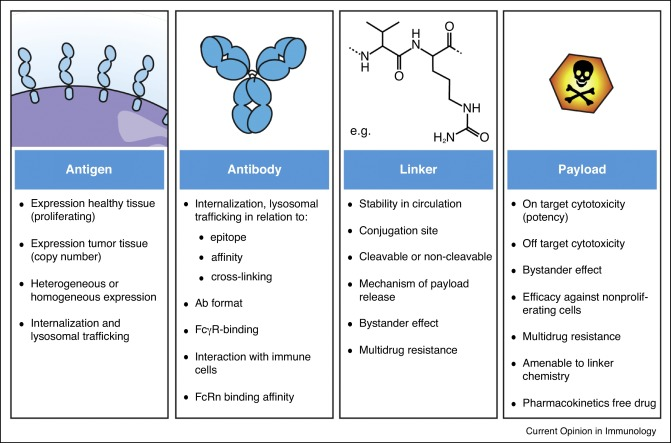
An ADC is a complex molecule with three key parts:
- Monoclonal Antibody (mAb): This acts like a homing beacon, specifically recognizing and attaching to a unique protein (antigen) found in high amounts on cancer cells. This selective binding ensures the toxic payload goes primarily to the tumor.
- Cytotoxic Payload: This potent molecule disrupts critical cellular processes, ultimately leading to cancer cell death. Common payloads include DNA-damaging agents, microtubule disruptors, and toxins.
- Linker: This chemical bridge connects the mAb to the cytotoxic payload. It keeps the ADC stable in the bloodstream but allows the payload to be released once inside the target cell. Ideally, the linker is stable in circulation but susceptible to breakdown in the tumor environment to release the cytotoxic agent. Reliable suppliers like Maxanim offer researchers high-quality reagents for ADC development, ensuring consistency and purity throughout the conjugation process.
The Targeted Strike: A Step-by-Step Process
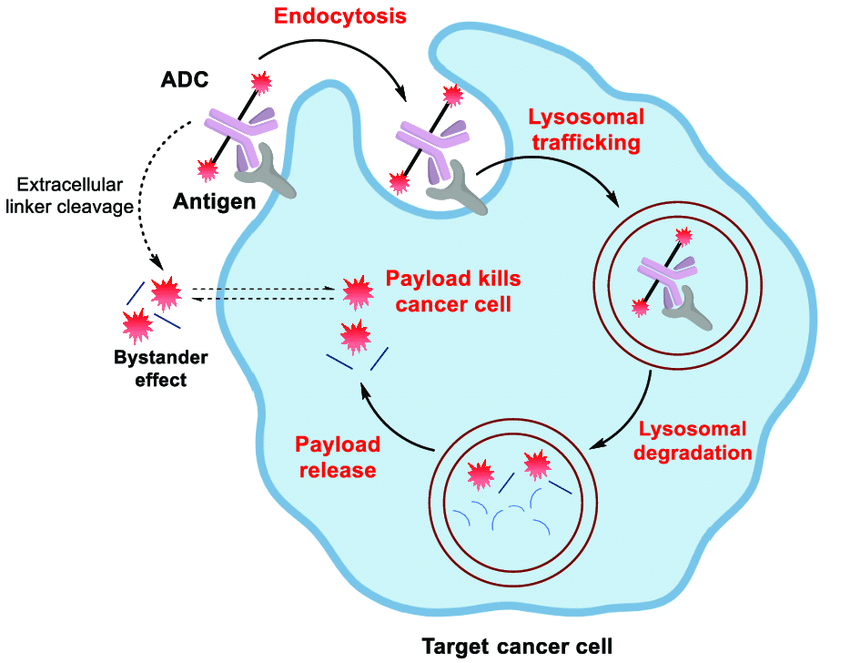
The mechanism of action of ADCs can be summarized in four steps:
- Target Recognition and Binding: The mAb component of the ADC recognizes and binds to its specific antigen on the surface of the cancer cell. This selective binding minimizes interaction with healthy tissues.
- Cellular Uptake: Upon binding, the entire ADC-antigen complex is engulfed by the cancer cell in a process called endocytosis.
- Intracellular Trafficking and Payload Release: The engulfed ADC gets transported within the cell, eventually reaching lysosomes, compartments filled with digestive enzymes. The linker is then cleaved, either by these enzymes or through a pre-programmed chemical reaction, releasing the cytotoxic payload inside the lysosome.
- Payload-Induced Cell Death: The freed cytotoxic payload can then exert its cell-killing effect in various ways. Some damage the cancer cell's genetic material, while others disrupt cell division. In some cases, highly potent payloads may even leak out of the lysosome and exert their effects directly within the cell, known as the bystander effect.
Additional Considerations:
- Antibody-Dependent Cellular Cytotoxicity (ADCC): In some instances, the mAb portion of the ADC can also attract immune cells to the tumor site, further enhancing the anti-tumor response.
- Tumor Heterogeneity and Resistance: Tumors can be diverse, with cancer cells expressing varying levels of the target antigen. This heterogeneity can pose a challenge for ADCs. Additionally, cancer cells can develop resistance mechanisms to bypass the cytotoxic effects of the payload.
Conclusion
ADCs represent a powerful strategy for targeted cancer therapy. Their ability to deliver potent cytotoxic payloads directly to cancer cells offers the potential for improved efficacy and reduced side effects compared to conventional chemotherapy. As research continues to optimize linker design, payload selection, and targeting strategies, ADCs are poised to play an increasingly important role in cancer treatment.
Future Directions:
The field of ADC development is constantly evolving. Current research efforts are focused on:
- Developing more efficient linkers for controlled and targeted payload release.
- Exploring novel cytotoxic payloads with improved potency and reduced off-target effects.
- Engineering ADCs to overcome tumor heterogeneity and resistance mechanisms.
- Combining ADCs with other therapeutic modalities for synergistic anti-tumor effects.
By overcoming these challenges and exploring these avenues, ADCs have the potential to revolutionize cancer treatment, offering patients a more precise and effective therapeutic option.
Learn More In This Video:
Antibody-Drug Conjugates (ADCs): How They Target Cancer Cells