Introduction
Cancer treatment has constantly improved, aiming for better effectiveness and reduced side effects for patients. Traditional chemotherapy has been widely used for a long time, but it affects healthy cells along with cancer cells, causing side effects like hair loss, nausea, and fatigue. Antibody-Drug Conjugates (ADCs) are a new type of targeted therapy that offers a more precise way to kill cancer cells. This article will compare and contrast ADCs with traditional chemotherapy in terms of how well they work, side effects, and patient outcomes.
Mechanism of Action
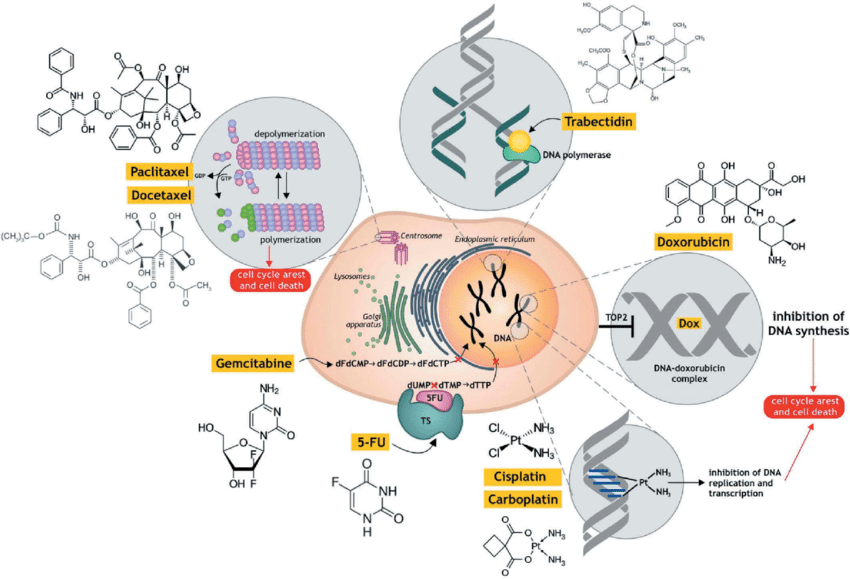
- Traditional Chemotherapy: These drugs target fast-growing cells, including cancer cells, by interfering with their division process. However, they can also harm healthy dividing cells, causing side effects.
-
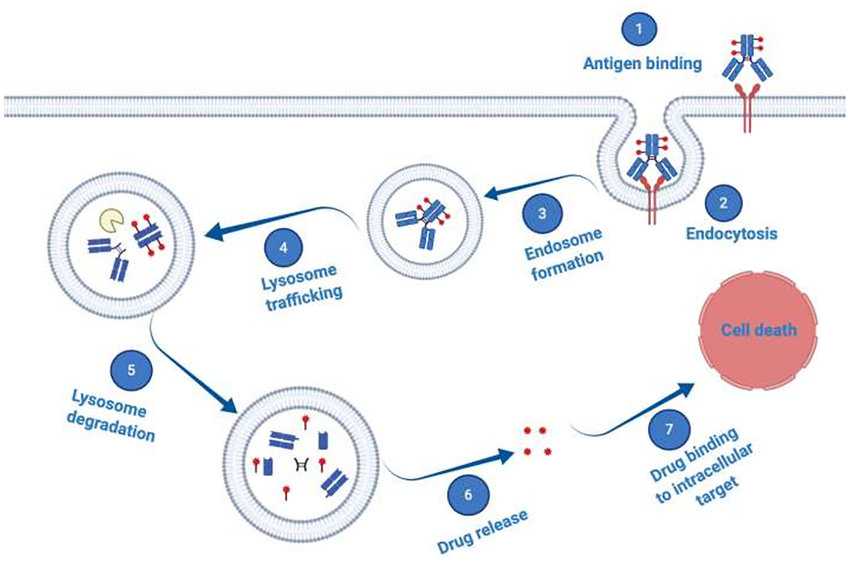
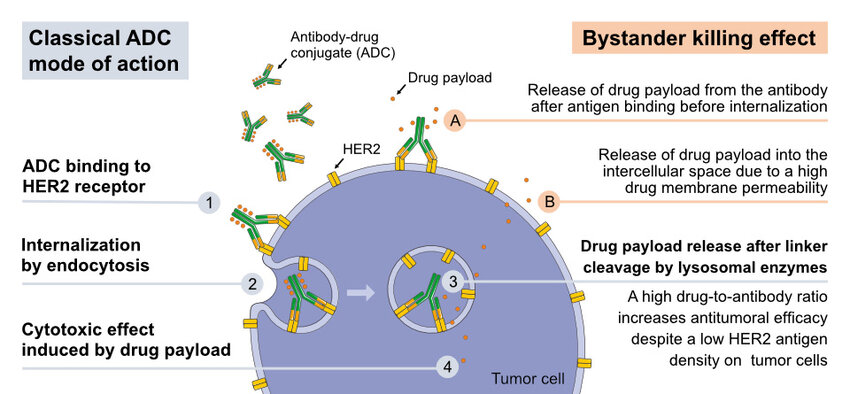
Antibody-Drug Conjugates (ADCs): ADCs have three main parts:
- Monoclonal Antibody: This molecule can identify and attach to a specific protein (antigen) on the surface of cancer cells.
- Cytotoxic Payload: A powerful chemotherapy drug that kills cancer cells.
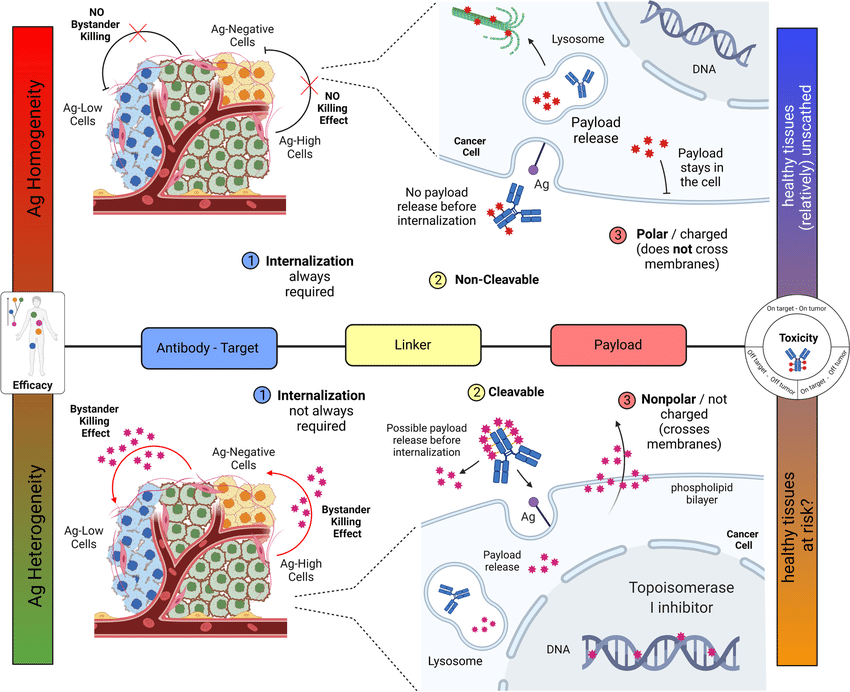
- Linker: A chemical chain that connects the antibody and the cytotoxic payload. Ideally, the linker should be stable in the bloodstream but break apart inside the tumor, releasing the cytotoxic drug.
When administered, the ADC uses the antibody to bind to cancer cells. The entire ADC is then taken up by the cell, and the linker releases the cytotoxic payload inside the tumor, maximizing its effect on cancer cells while minimizing exposure of healthy tissues to the drug.
Efficacy
Clinical trials have shown promising results for ADCs in treating various cancers, including breast, lung, and HER2-positive stomach cancers. ADCs can be just as effective or even more effective than traditional chemotherapy for certain groups of patients, especially those with tumors that have the targeted antigen.
For instance, a study by showed that Kadcyla (ado-trastuzumab emtansine), an ADC that targets HER2 receptors, significantly improved progression-free survival compared to standard chemotherapy in patients with HER2-positive metastatic breast cancer.
Side Effects
A major advantage of ADCs is their targeted approach. By minimizing exposure of healthy tissues to the cytotoxic payload, ADCs may cause less severe and frequent side effects compared to traditional chemotherapy.
However, ADCs can still cause side effects. Depending on the specific antibody, linker, and payload combination, patients may experience fatigue, nausea, and neutropenia (low white blood cell count). Additionally, the targeted antigen itself may be present on healthy tissues, leading to specific side effects.
Patient Outcomes
The targeted nature of ADCs has the potential to improve patient quality of life by reducing side effects from treatment. Additionally, some ADCs are more effective than traditional chemotherapy, potentially leading to better overall survival rates for specific patient populations.
It is important to note that ADCs are often more expensive than traditional chemotherapeutic agents. Furthermore, ongoing research is essential to improve linker design, enhance payload potency, and identify new targets for broader application of ADCs. Researchers around the world, along with suppliers of high-quality reagents like Maxanim, are continually working to advance ADC development for the benefit of patients.
Learn More In This Video:
Conclusion
ADCs represent a significant advancement in cancer treatment, offering a more targeted approach with potentially fewer side effects compared to traditional chemotherapy. While ongoing research is required to optimize their efficacy and affordability, ADCs hold immense promise for improved patient outcomes and a more personalized approach to cancer therapy.
Antibody-Drug Conjugates (ADCs) vs. Traditional Chemotherapy: Targeted Therapy vs. Broad Spectrum Treatment