Immunotherapy has revolutionized cancer treatment by harnessing the body's own immune system to combat tumors. However, a significant portion of patients exhibit variable responses, highlighting the need for personalized treatment strategies. Recent research suggests that the composition of gut microbiota, the trillions of microorganisms residing in the gastrointestinal tract, plays a crucial role in modulating the efficacy of immunotherapy.
Gut Microbiota and the Immune System
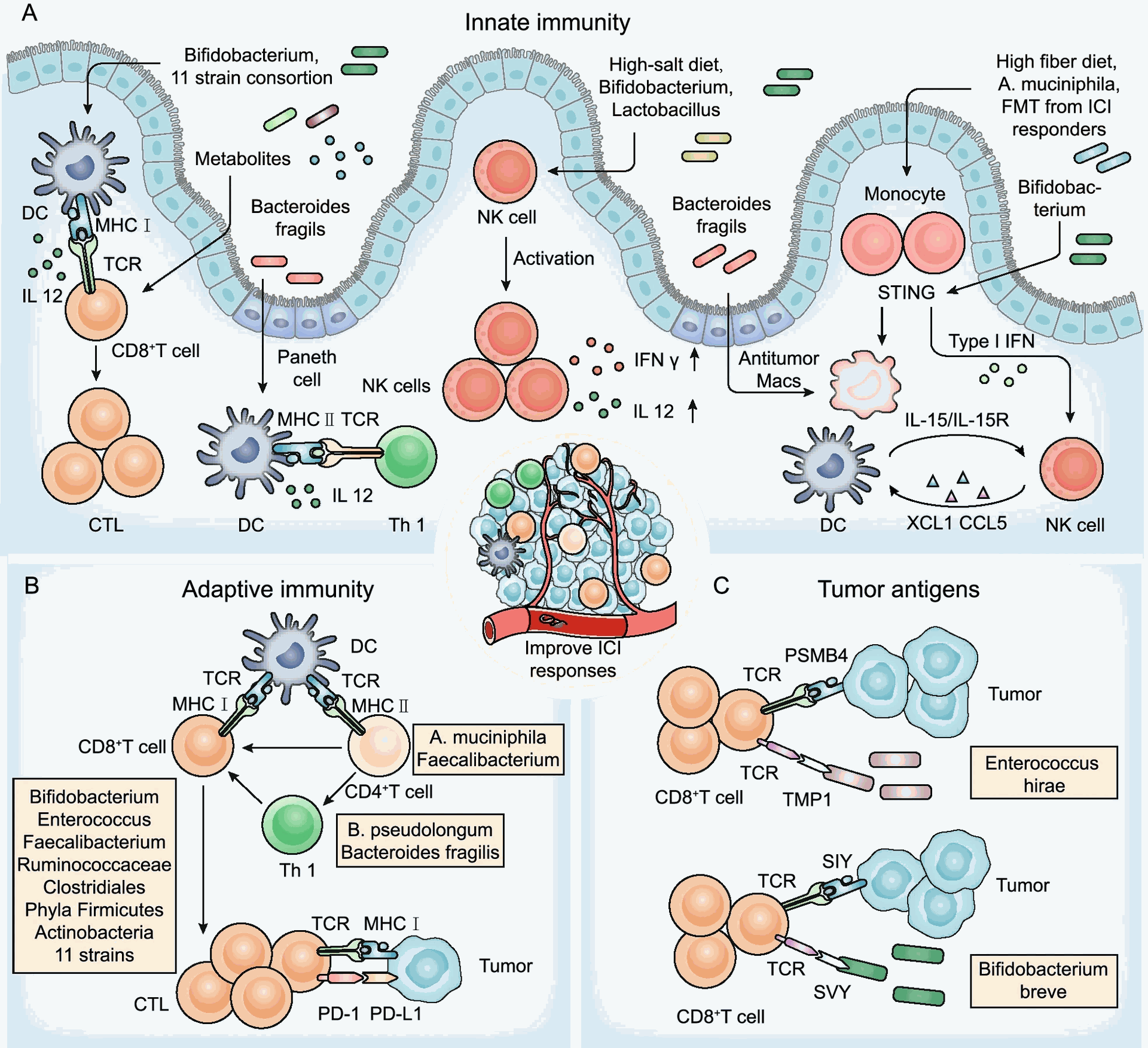
The gut microbiota maintains a symbiotic relationship with the host, influencing various physiological processes, including immune function. Specific bacterial communities promote immune homeostasis by stimulating the maturation and function of immune cells, such as dendritic cells (DCs) and T lymphocytes. Conversely, dysbiosis, an imbalance in gut microbial composition, can trigger chronic inflammation and impair anti-tumor immunity.
Gentaur Group offers a complete selection of components specifically designed for research in this field.
Mechanisms of Modulation
Studies suggest that gut microbiota can influence immunotherapy response through several mechanisms:
- Modulation of antigen presentation: Specific gut microbes can metabolize dietary components into metabolites that activate DCs, enhancing their ability to present tumor antigens to T cells, thereby priming the adaptive immune response.
- Regulation of T cell subsets: Certain bacterial species can promote the expansion and activation of effector T cells (cytotoxic T lymphocytes, Th1 cells) while suppressing regulatory T cells (Tregs) that dampen anti-tumor immunity.
- Shaping the tumor microenvironment (TME): Gut microbiota can influence the TME by modulating the infiltration and function of immune cells within the tumor. Some bacteria can induce the production of pro-inflammatory cytokines and chemokines, leading to increased recruitment of cytotoxic T cells into the tumor site
Clinical Evidence
Emerging clinical data support the link between gut microbiota and immunotherapy response. Studies have shown that patients with a more diverse and beneficial gut microbiota composition tend to have better clinical outcomes with immunotherapy compared to those with a depleted or dysbiotic microbiota. Conversely, antibiotic use, which disrupts gut microbial communities, has been associated with reduced efficacy of immunotherapy in some patients.
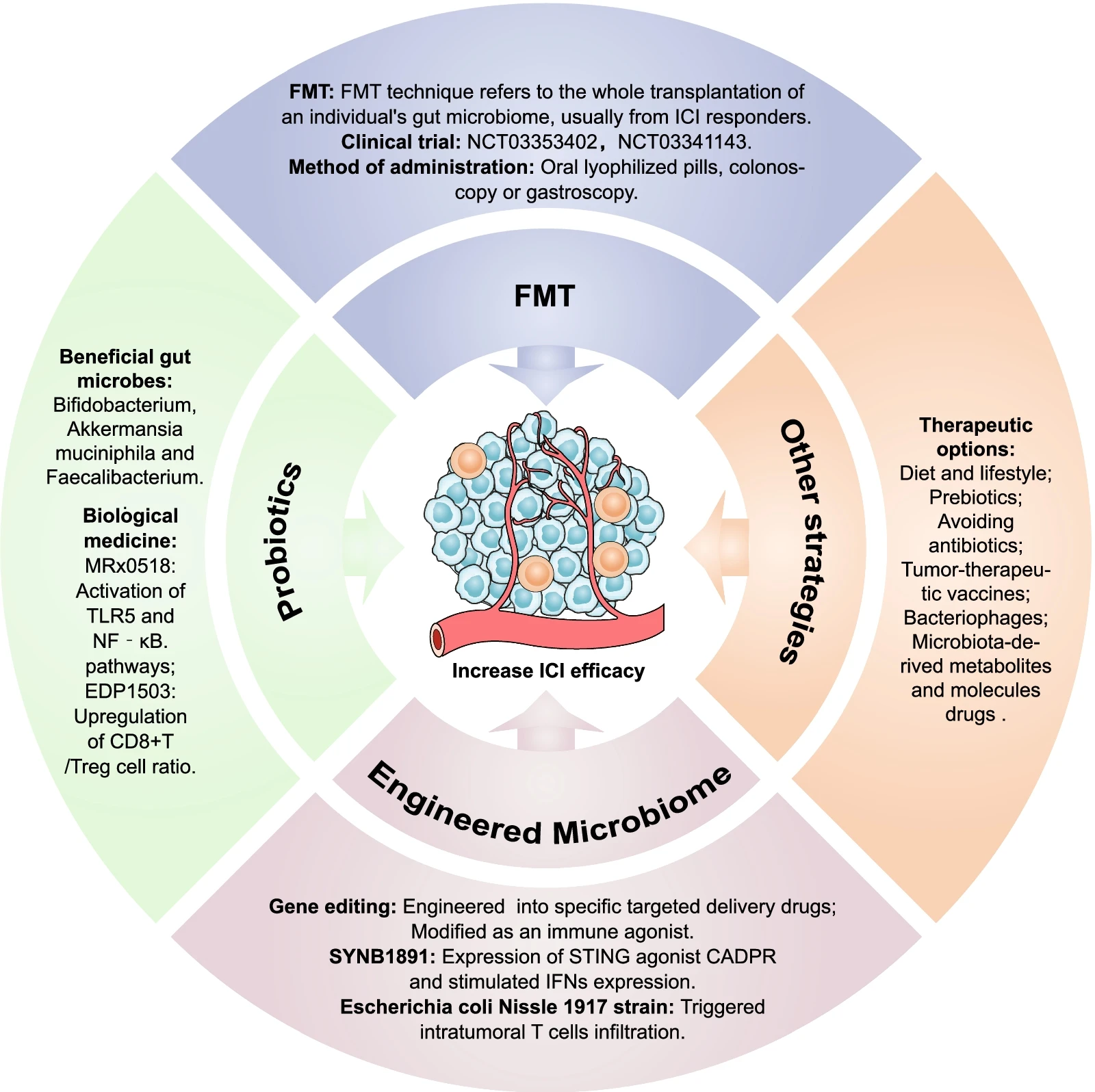
Fecal Microbiota Transplantation (FMT) and the Future
Fecal microbiota transplantation (FMT), the transfer of healthy gut microbiota from a donor to a recipient, is being explored as a potential strategy to manipulate the gut microbiome and improve immunotherapy response. Early-phase clinical trials have shown promising results, with FMT leading to increased response rates and reduced immune-related adverse events in some patients.
Conclusion
The gut microbiota represents a novel and exciting target for optimizing immunotherapy efficacy. By elucidating the intricate interplay between gut microbes, the immune system, and tumor biology, researchers can develop strategies to manipulate the gut microbiome to enhance patient response and personalize cancer treatment. Future research will focus on identifying specific bacterial signatures associated with improved immunotherapy outcomes, refining FMT protocols, and exploring the development of prebiotics and probiotics to promote a beneficial gut microbiota for cancer patients.
For a closer look, watch "Gut Responses: Microbes, Inflammation, and Cancer" below.

Gut Microbiota and Immunotherapy Response