Immunotherapy has revolutionized cancer treatment by leveraging the body's immune system to fight tumors. However, for many patients, immunotherapy alone might not be sufficient. Combining immunotherapy with established therapies like chemotherapy and radiation offers a promising approach to improve treatment effectiveness and overcome resistance. This enhanced efficacy stems from the combined effects of each treatment modality.
Immunotherapy and Chemotherapy
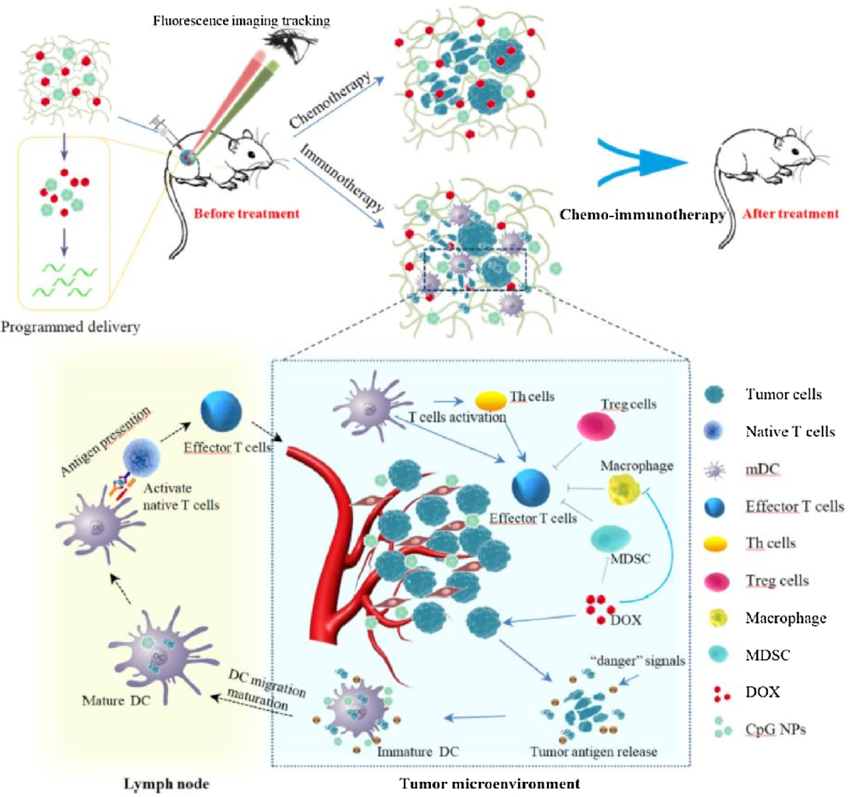
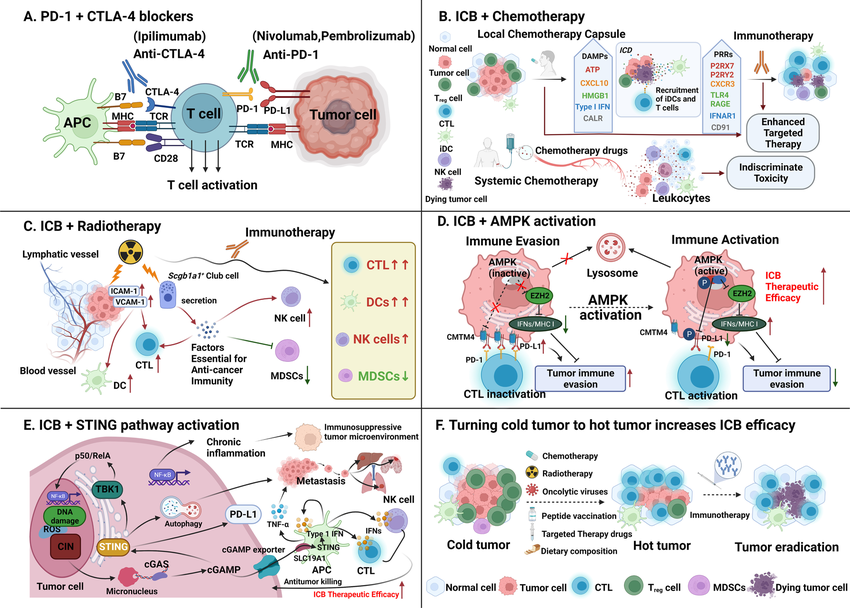
Chemotherapy effectively reduces tumor burden but can also suppress the immune system. Certain chemotherapy drugs can counteract this immunosuppression by stimulating the immune system. For instance, cyclophosphamide, a common chemotherapy agent, depletes regulatory T cells (Tregs) within the tumor microenvironment, allowing for a stronger anti-tumor immune response when combined with immunotherapy. Additionally, chemotherapy can induce tumor cell death in a way that releases tumor-specific antigens. These antigens are then taken up by antigen-presenting cells (APCs) and presented to the immune system for recognition and attack, further enhancing the effectiveness of immune checkpoint inhibitors (ICIs) – a major immunotherapy class that reactivates exhausted T cells.
Immunotherapy and Radiation Therapy
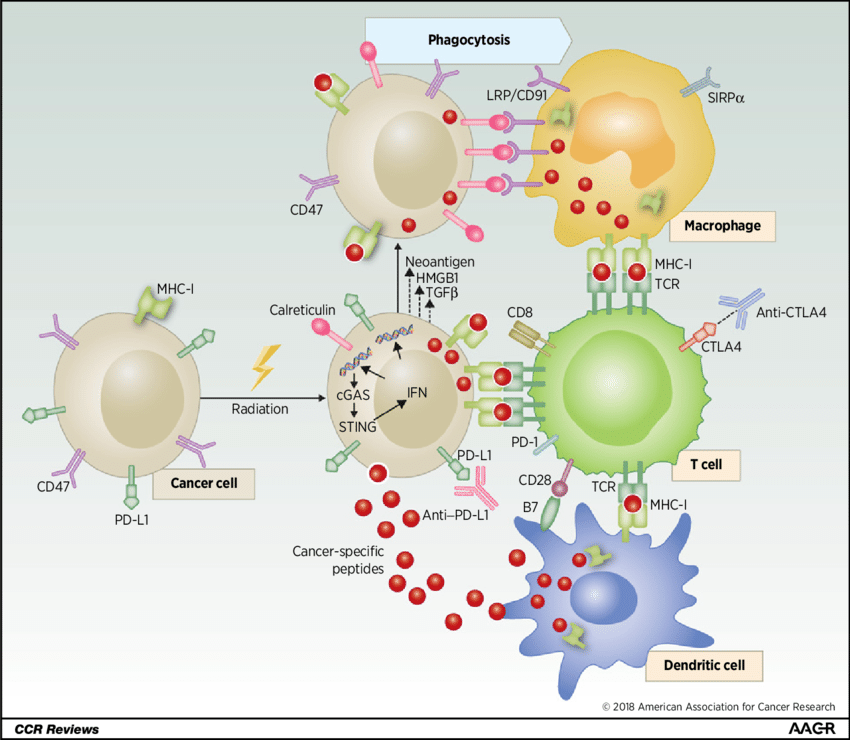
Radiation therapy primarily targets tumor cells directly, causing DNA damage and cell death. However, it can also exert immunomodulatory effects that work synergistically with immunotherapy. Similar to chemotherapy, radiation can promote the release of tumor antigens, making tumor cells more susceptible to immune recognition. Furthermore, radiation can increase the expression of Major Histocompatibility Complex (MHC) molecules on tumor cells, facilitating antigen presentation to T cells. Additionally, radiation therapy can reshape the tumor microenvironment by eliminating immunosuppressive cells and promoting the infiltration of effector T cells, creating a more favorable environment for immunotherapy to function.
Combinations with Other Treatment Modalities
The potential of immunotherapy extends beyond traditional approaches. Ongoing research explores the synergistic effects of immunotherapy with targeted therapies that inhibit specific cancer-causing pathways. These targeted therapies can modify the tumor microenvironment, making it more conducive to T cell infiltration and activation. Additionally, CAR T-cell therapy, a personalized form of immunotherapy that engineers a patient's T cells to recognize and attack cancer cells, shows promise when combined with other therapies like immune checkpoint blockade.
Challenges and Future Directions
Despite promising preclinical and clinical data, combining immunotherapy with other modalities presents challenges. A major hurdle is the potential for increased toxicity due to the additive or synergistic effects of combination therapies. Careful optimization of treatment regimens and patient selection are crucial to minimize these side effects. Additionally, identifying predictive biomarkers to guide treatment decisions and identify patients who are most likely to benefit from combination therapy remains an ongoing area of investigation.

Future research efforts require continued investigation into optimizing treatment regimens and identifying biomarkers. Additionally, the development of high-quality research reagents is essential for these advancements. Companies like Maxanim (Gentaur Group), a supplier of research reagents, can play a vital role by providing researchers with the tools they need to unlock the full potential of combination therapies in cancer treatment.
Conclusion
Combining immunotherapy with other cancer treatments holds immense promise for improving patient outcomes. By leveraging the strengths of each approach, we can create a more potent and multifaceted attack on cancer cells. Continued research efforts focused on optimizing treatment regimens, identifying predictive biomarkers, and exploring novel combinations will be essential to fully realize the potential of this therapeutic strategy.
Immunotherapy Combinations for Enhanced Cancer Treatment