Abstract:
Cancer immunotherapy has revolutionized cancer treatment by leveraging the immune system to fight tumors. Oncolytic viruses (OVs) are genetically modified viruses specifically designed to infect and replicate within cancer cells. This replication process directly destroys cancer cells while simultaneously stimulating an anti-tumor immune response. This article explores the potential of combining OVs with other established immunotherapies to improve treatment efficacy for a broader range of cancers.
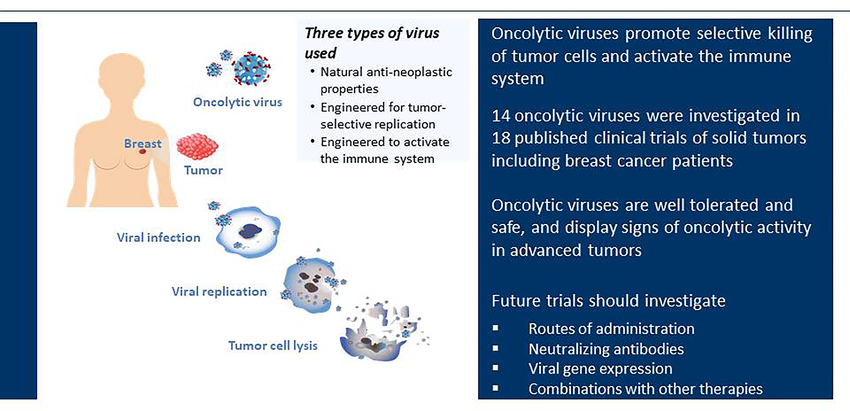
Oncolytic Viruses and Their Mechanism of Action:
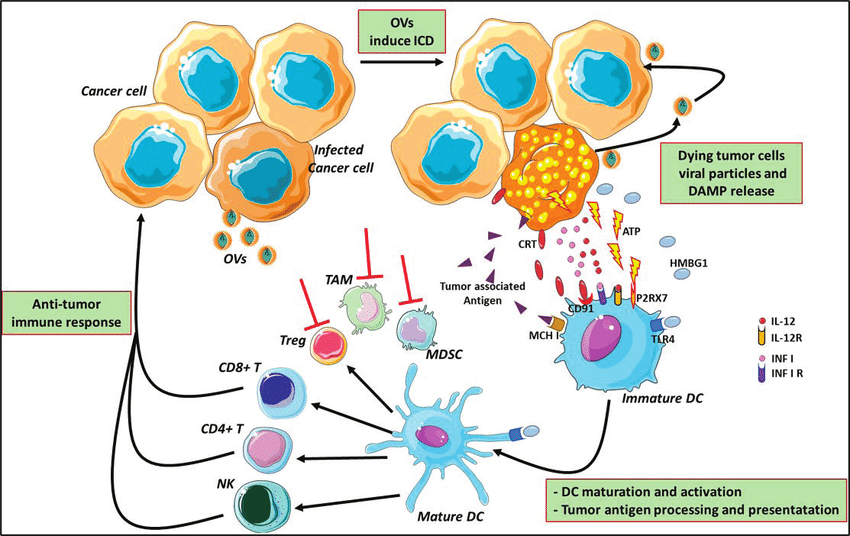
OVs are engineered to target and infect cancer cells selectively. Once inside, they replicate, causing the cancer cell to burst (lyse), leading to direct tumor cell death. Additionally, OVs trigger a specific type of cell death (immunogenic cell death) that releases tumor antigens and danger signals. These signals alert the immune system to the presence of cancer cells, promoting an immune response against the tumor.
Synergy with Immunotherapy:
The ability of OVs to activate the immune system creates a unique opportunity for combination therapy with existing immunotherapies:
- Immune Checkpoint Inhibitors (ICIs): ICIs, such as anti-PD-1 and anti-CTLA-4 antibodies, work by removing inhibitory signals within the tumor microenvironment (TME), allowing immune cells to attack the tumor. OVs can enhance the effectiveness of ICIs by:
- Increasing the number of activated T cells infiltrating the tumor.
- Converting tumors with low immune cell infiltration ("cold" tumors) into tumors with high immune cell infiltration ("hot" tumors), making them more susceptible to ICI therapy.
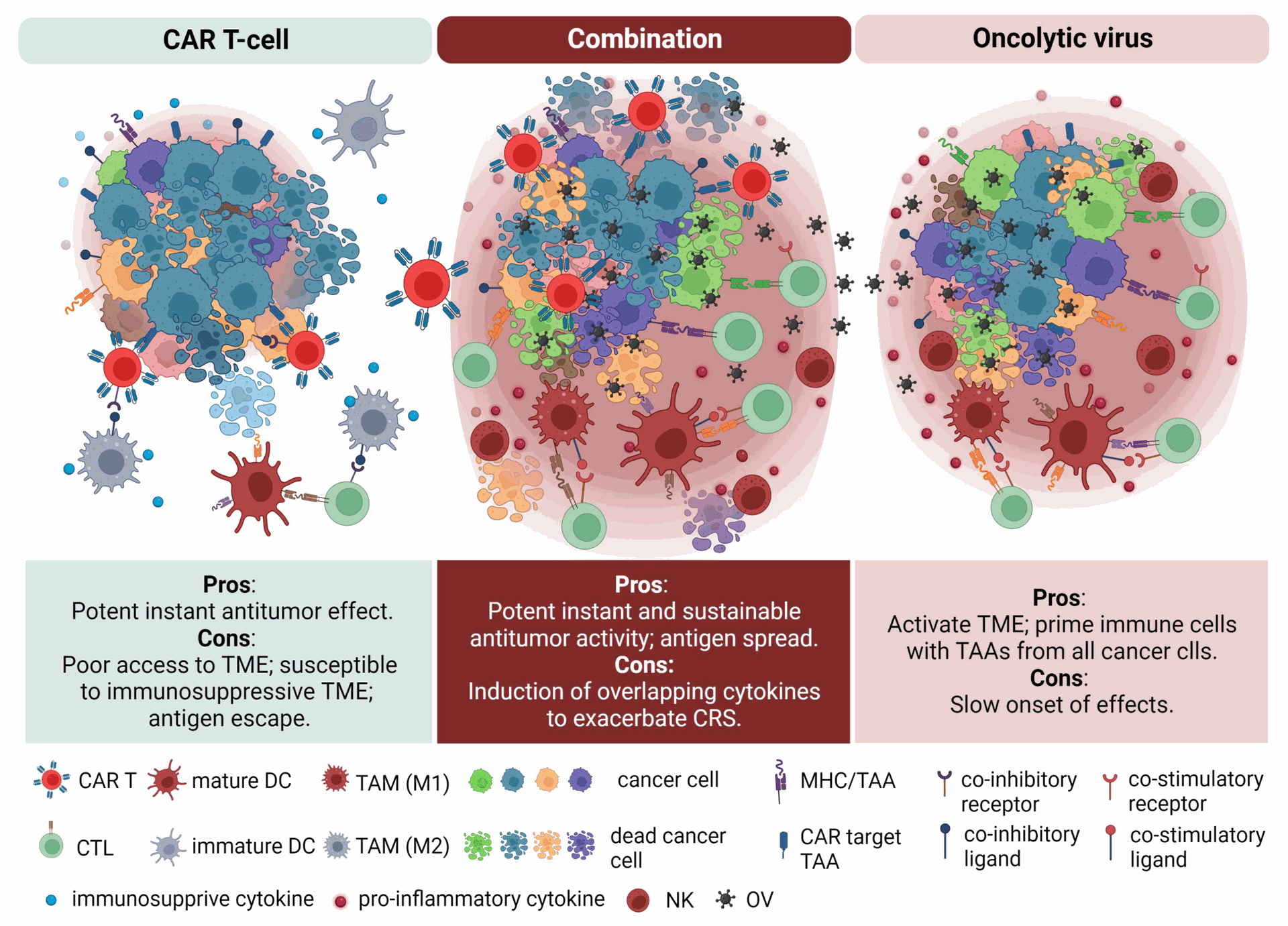
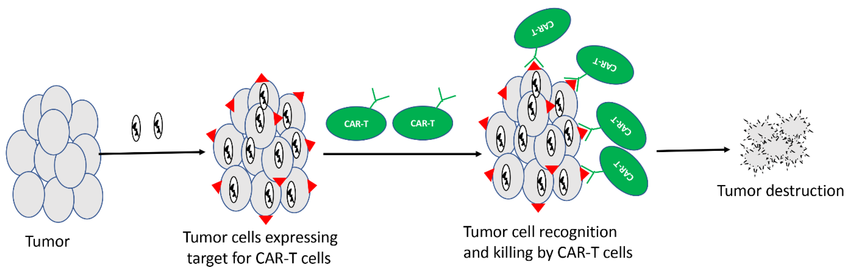
- CAR T-cell Therapy: Chimeric antigen receptor (CAR) T-cell therapy utilizes genetically engineered T cells to target and eliminate cancer cells. OVs can:
- Improve the expansion and persistence of CAR T cells within the TME.
- Enhance the migration of CAR T cells towards tumors.
- Cytokines: Cytokines, such as IL-2, stimulate the proliferation and activation of immune effector cells. OVs can work together with cytokines by creating a more favorable environment within the TME for cytokine action.
Optimizing Oncolytic Virus Therapy:
Researchers are actively investigating methods to improve the effectiveness of OV therapy. This includes optimizing viral design for:
- Enhanced tumor specificity: Ensuring that the virus only infects cancer cells and minimizes infection of healthy cells.
- Increased potency: Improving the efficiency of viral replication and subsequent tumor cell death.
Reliable Research Reagents are Essential:
High-quality research reagents are crucial throughout the development process for successful OV therapy. Companies like Maxanim provide researchers with essential tools for OV design, vector engineering, and pre-clinical testing.
Clinical Trials and Future Directions:
Several clinical trials are underway to evaluate the safety and efficacy of combining OVs with various immunotherapies. Early results are promising, showing improved patient outcomes compared to using either therapy alone.
Future research directions include:
- Identifying biomarkers to predict which patients will respond best to combination therapy.
- Developing strategies to overcome potential limitations, such as pre-existing immunity to the chosen OV.
Conclusion:
The combination of OVs with immunotherapy represents a promising new approach for cancer treatment. By exploiting the complementary mechanisms of each therapy, this strategy has the potential to overcome resistance to current therapies and improve outcomes for a wider range of cancer patients.
Oncolytic Viruses: A Novel Approach to Cancer Immunotherapy