Immunotherapy has revolutionized cancer treatment, harnessing the power of the immune system to combat tumors. However, a key challenge remains – predicting which patients will benefit most from this therapy. Biomarkers, measurable biological indicators, offer a promising avenue for personalized treatment selection.
Established Biomarkers for Immunotherapy Response
Three primary biomarkers have garnered significant attention:
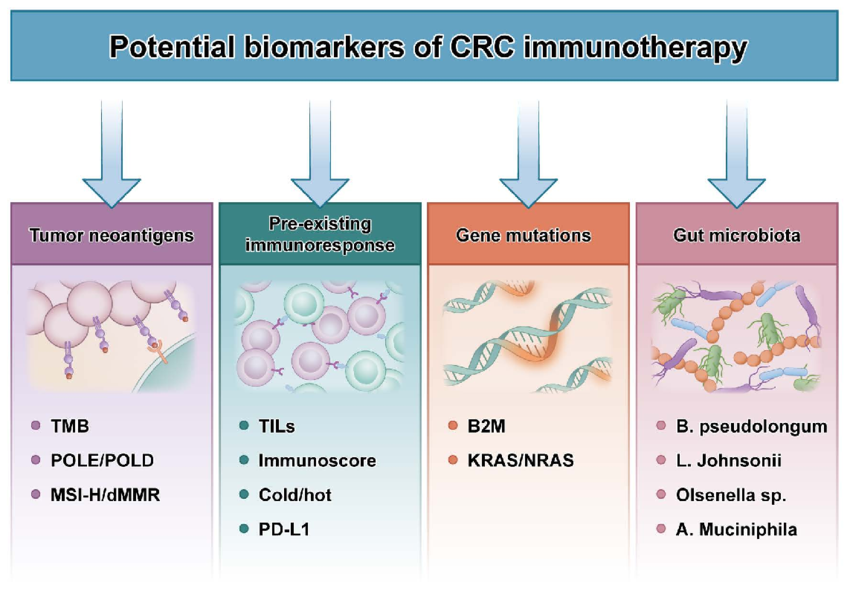
- Programmed death-ligand 1 (PD-L1): A protein expressed on tumor cells and antigen-presenting cells. Its interaction with PD-1 on T cells suppresses immune response. High PD-L1 expression often correlates with favorable response to immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 pathway. However, PD-L1 expression can be heterogeneous within tumors, and its cut-off for positivity varies across cancer types, highlighting limitations in its standalone use.
If you are working in this research field, all the components can be supplied by Gentaur Group.
- Microsatellite instability (MSI)/Defective mismatch repair (dMMR): These arise from errors in DNA repair mechanisms, leading to high mutation burdens and the generation of unique neoantigens (foreign molecules) recognized by the immune system. Patients with MSI/dMMR tumors, particularly in colorectal and endometrial cancers, demonstrate exceptional responses to ICIs due to robust immune activation by neoantigens.
- Tumor mutational burden (TMB): Represents the number of mutations per megabase of DNA in a tumor. High TMB signifies increased neoantigen load, potentially predicting response to ICIs across various cancer types. However, similar to PD-L1, TMB cut-offs for response vary, and its utility in specific cancers requires further investigation.
Emerging Biomarkers:
The field actively explores additional promising biomarkers:
- Tumor-infiltrating lymphocytes (TILs): The presence and type of immune cells within the tumor microenvironment can influence response. High levels of CD8+ T cells, cytotoxic immune cells, are associated with improved outcomes in certain cancers.
- Peripheral blood biomarkers: Analysis of immune cell subsets, circulating tumor DNA, and soluble factors in blood offers a non-invasive approach. However, these markers require further validation and may need to be evaluated in conjunction with tumor-based biomarkers.
- Gene expression signatures: Studying the expression profiles of specific genes associated with immune response may provide insights into a patient's suitability for immunotherapy.
Challenges and Opportunities in Biomarker Development
Despite significant progress, challenges persist:
- Standardization: Variability in assay methods for established biomarkers like PD-L1 necessitates standardized protocols for accurate and reproducible results.
- Integration of Multimodal Data: Combining information from multiple biomarkers, including tumor characteristics and immune response profiles, may hold the key for robust response prediction.
- Understanding Mechanisms of Resistance: Identifying mechanisms by which tumors evade immune attack is crucial for developing strategies to overcome resistance and improve patient outcomes.
Conclusion
Biomarkers hold immense potential for optimizing patient selection for immunotherapy. Ongoing research focused on standardization, integration of diverse data points, and elucidating resistance mechanisms will pave the way for a future of personalized immunotherapy, maximizing patient benefit and minimizing unnecessary treatment exposure.
See a video explainer below!
![The biological steps to achieve an effective immune response in NPC. This figure is based on the original cancer–immunity cycle proposed by Chen and Mellman. 13 The steps and factors affecting the response of ICIs are edited to reflect the specific immunobiology of NPC. The release of NPC-specific antigens (1) and their subsequent presentation by dendritic cells (DCs) and other antigen-presenting cells (APCs) (2), is followed by T-cell priming and activation (3), trafficking into tumors (4), tumor infiltration (5), and recognition and killing of tumor cells (6 and 7). Notable NPC-specific characteristics of this cycle favoring antitumor immunity include the release of NPC-specific antigens (including EBV-derived antigens and a large number of neoantigens), activated monocytes which could also be considered as a kind of APCs, promoting the trafficking of immune cells into tumors [via tumor inflammasomes, increased chemoattraction, notable interferon-α (IFN-α) and IFN-γ], dense infiltration of immune cells into tumors, high expression of inhibitory receptors, elevated cytotoxicity genes, and chemokine receptors. Conversely, antitumor immunity could be impeded by the loss of NPC-specific neoantigens, major histocompatibility complex protein (MHC) aberration, defective DCs or antigen-processing machinery components (APM) deficiency, high epithelial indoleamine 2,3-dioxygenase, abundant myeloid-derived suppressor cells (MDSC) or regulatory T-cells (Tregs), rich M2-type tumor-associated macrophages (TAMs), and several features typically associated with T-cell exhaustion. The biological steps to achieve an effective immune response in NPC. This figure is based on the original cancer–immunity cycle proposed by Chen and Mellman. 13 The steps and factors affecting the response of ICIs are edited to reflect the specific immunobiology of NPC. The release of NPC-specific antigens (1) and their subsequent presentation by dendritic cells (DCs) and other antigen-presenting cells (APCs) (2), is followed by T-cell priming and activation (3), trafficking into tumors (4), tumor infiltration (5), and recognition and killing of tumor cells (6 and 7). Notable NPC-specific characteristics of this cycle favoring antitumor immunity include the release of NPC-specific antigens (including EBV-derived antigens and a large number of neoantigens), activated monocytes which could also be considered as a kind of APCs, promoting the trafficking of immune cells into tumors [via tumor inflammasomes, increased chemoattraction, notable interferon-α (IFN-α) and IFN-γ], dense infiltration of immune cells into tumors, high expression of inhibitory receptors, elevated cytotoxicity genes, and chemokine receptors. Conversely, antitumor immunity could be impeded by the loss of NPC-specific neoantigens, major histocompatibility complex protein (MHC) aberration, defective DCs or antigen-processing machinery components (APM) deficiency, high epithelial indoleamine 2,3-dioxygenase, abundant myeloid-derived suppressor cells (MDSC) or regulatory T-cells (Tregs), rich M2-type tumor-associated macrophages (TAMs), and several features typically associated with T-cell exhaustion.](/web/image/41500-d96c7567/image.png?access_token=efe6e67f-8845-4607-a906-c2e60a20e104)
Optimizing Immunotherapy: Biomarkers for Personalized Treatment