Proteins are essential cellular molecules with specific functions that rely on their precise three-dimensional structure. When protein folding goes wrong, they misfold and clump together into toxic assemblies. These aggregates disrupt cellular balance, leading to a series of harmful consequences. Understanding how misfolded proteins affect cellular function is vital for uncovering the causes of various diseases.
Mechanisms of Toxicity:
- Loss of Activity: Misfolded proteins often become inactive. If these proteins are critical for cellular processes, their dysfunction can cripple the cell. In Alzheimer's disease, for example, β-amyloid aggregation disrupts communication between neurons.
- Toxic Gain of Function: In some cases, misfolded proteins acquire new toxic properties. These abnormal proteins can interact with other cellular components, causing dysfunction and cell death. Prions, infectious agents composed solely of misfolded proteins, are an example and can trigger neurodegeneration.
- Proteostasis Disruption: Cells have a quality control system called proteostasis that ensures proper protein folding and eliminates misfolded ones. Toxic protein assemblies can overload this system, leading to a collapse in proteostasis. This further worsens protein misfolding and aggregation, creating a vicious cycle.
Cellular Consequences:
- Organelle Dysfunction: Protein aggregates can accumulate in various cellular compartments, disrupting their normal function. For instance, misfolded proteins in the endoplasmic reticulum (ER) can trigger ER stress, impairing protein synthesis and leading to cell death.
- Oxidative Stress: Misfolded proteins can generate reactive oxygen species (ROS), which damage cellular components like DNA and membranes through oxidative stress.
- Inflammation: The cellular response to protein aggregates often involves inflammatory pathways. Chronic inflammation can further contribute to tissue damage and disease progression.
For scientists, I invite you to explore our extensive catalog of research materials specifically designed to support your investigations into protein aggregation.
Diseases Associated with Protein Misfolding:
- Neurodegenerative Diseases: Alzheimer's disease, Parkinson's disease, Huntington's disease, and Amyotrophic Lateral Sclerosis (ALS) involve the aggregation of specific proteins in the brain.
- Proteinopathies: These diseases are caused by misfolded protein deposition in various organs, including cystic fibrosis and Gaucher's disease.
- Systemic Amyloidoses: In these disorders, amyloid fibrils, a specific type of protein aggregate, accumulate throughout the body, damaging tissues and organs.
Conclusion:
Misfolded protein assemblies have a profound impact on cellular function. Understanding how these aggregates exert their detrimental effects is crucial for developing treatments for protein misfolding diseases. Current research focuses on promoting proper protein folding, preventing aggregation, and enhancing clearance of toxic assemblies.
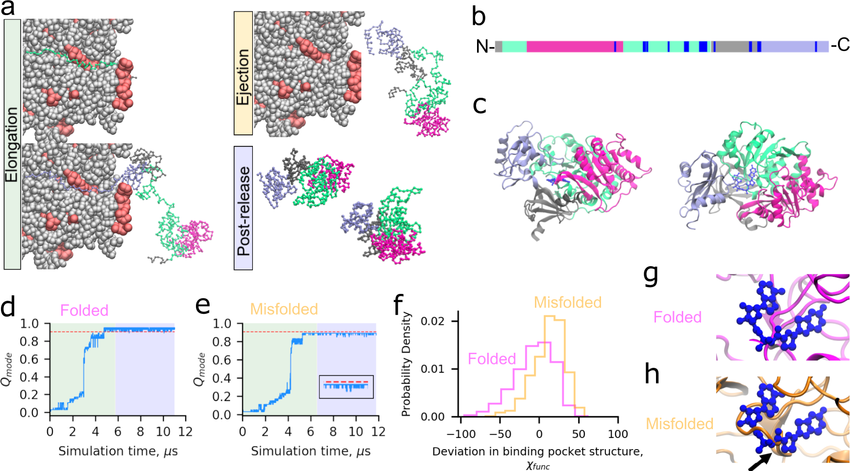
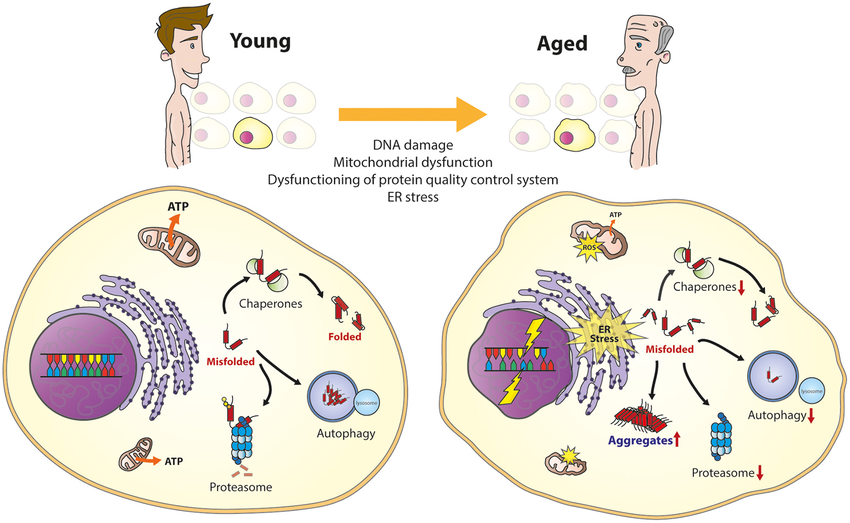
Protein Misfolding and Cellular Dysfunction