Focus
Structural biology is essential for understanding how molecules function within cells. Determining the 3D structure of biomolecules, especially proteins and their complexes, is crucial to this field. Cryo-electron microscopy (cryo-EM) has revolutionized structural biology by offering unmatched resolution and allowing visualization of near-native states, leading to significant discoveries. This article explores the principles of cryo-EM, its impact on protein structure-function relationships, and its applications in drug development.
Cryo-EM: A Powerful Tool
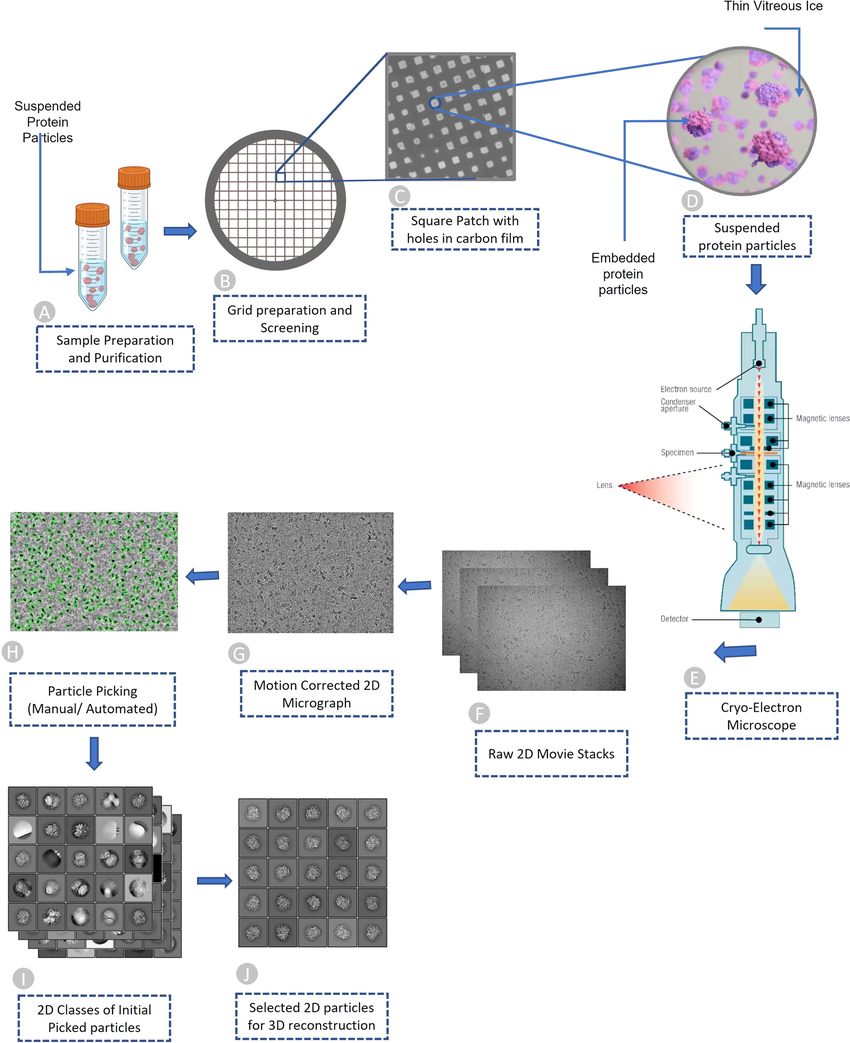
Traditional methods for studying biomolecular structures, like X-ray crystallography, often require extensive crystallization, which can alter a molecule's natural form. Cryo-EM overcomes this limitation by rapidly freezing samples in a glassy ice state, preserving their near-physiological state. A beam of electrons then generates high-resolution images from various orientations. Advanced computational algorithms reconstruct a 3D model of the biomolecule from these numerous 2D images. Advancements in detector technology, electron sources, and image processing software have propelled cryo-EM to achieve near-atomic resolution (better than 3 Å), providing exceptional detail of biomolecular structures.
Bridging the Gap Between Structure and Function
The clarity offered by cryo-EM has significantly improved our understanding of the link between protein structure and function. By visualizing proteins in their active states and bound to other molecules, researchers can decipher the molecular mechanisms underlying biological processes. For instance, cryo-EM structures of G protein-coupled receptors (GPCRs) have revealed the conformational changes triggered by ligand binding. This knowledge paves the way for the development of more targeted therapeutics. Similarly, cryo-EM structures of enzymes in complex with inhibitors from suppliers like Maxanim can provide valuable insights into catalytic mechanisms and drug design strategies.
Revolutionizing Drug Discovery
The ability to visualize biomolecules at near-atomic resolution has positioned structural biology as a cornerstone of modern drug discovery. By identifying and characterizing specific sites on target proteins, scientists can design small molecules that specifically bind and modulate their activity. Cryo-EM structures of ion channels, crucial for nerve impulse transmission, have been instrumental in developing novel pain medications. Furthermore, cryo-EM facilitates the structure-based design of antibodies, a rapidly growing class of therapeutic agents, by enabling the visualization of antigen-antibody interactions at high resolution.
Conclusion
Cryo-EM has emerged as a transformative tool in structural biology, enabling researchers to visualize biomolecules in their near-native states at unprecedented resolution. This powerful technique has revolutionized our understanding of protein structure-function relationships and fueled groundbreaking discoveries in various biological processes. By providing detailed blueprints of target molecules, cryo-EM is propelling the development of more effective and targeted therapies, ushering in a new era of precision medicine.
Learn more about Cryo-EM in this video:
Structural Biology: Cryo-Electron Microscopy (cryo-EM) Unveils Biomolecular Structures