T-cell receptor (TCR)-engineered T cell therapy is a new frontier in cancer treatment. It leverages the immune system's ability to recognize and destroy specific targets. TCR-T cells offer the potential for precise and lasting elimination of cancer cells. This blog post will explore the mechanisms of TCR-T cell therapy, its advantages and limitations, and the ongoing advancements in this field.
How TCR-T Cells Work
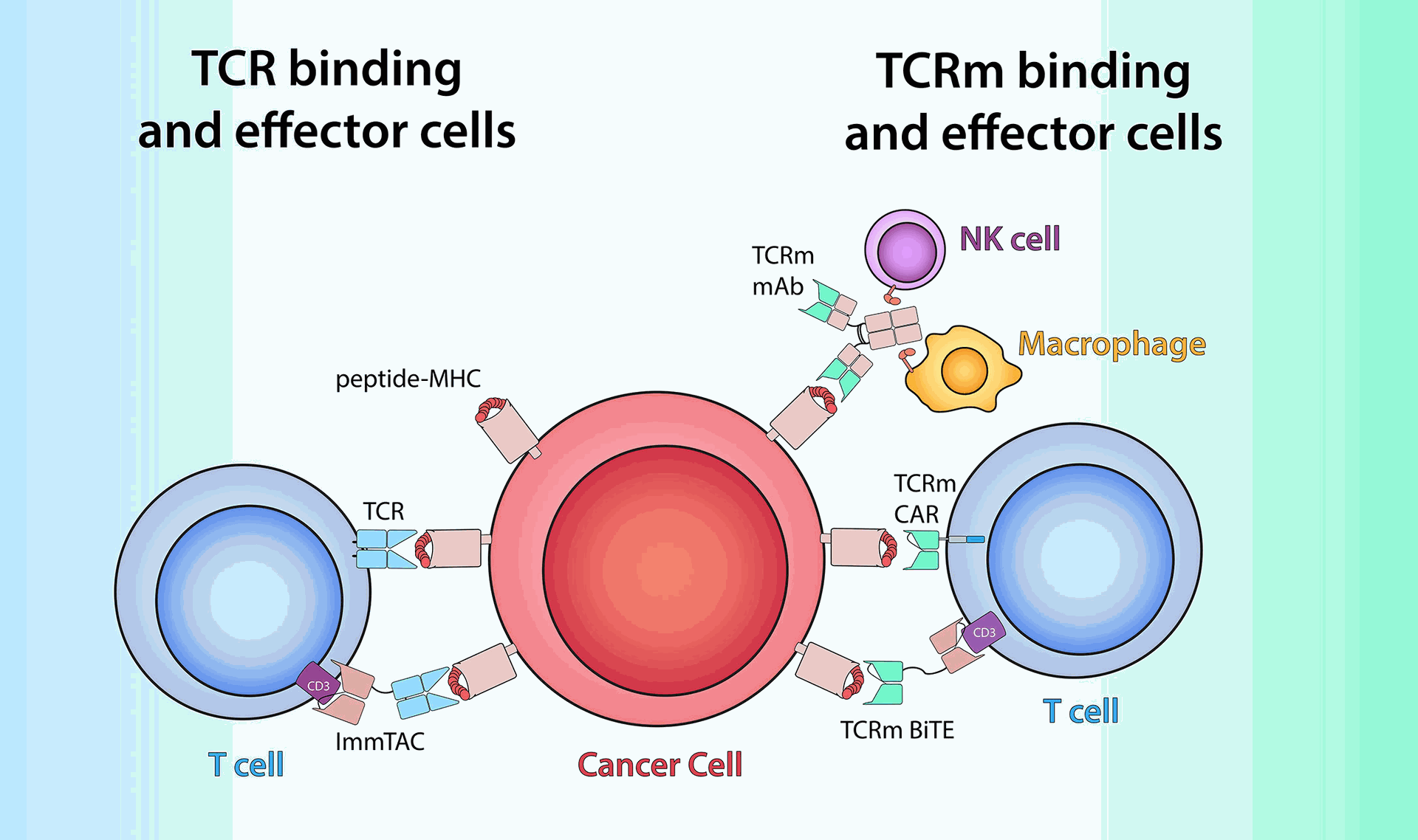
Unlike chimeric antigen receptor (CAR)-T cell therapy, which targets surface proteins on cancer cells, TCR-T cells target fragments of tumor-associated antigens (TAAs) that reside inside cancer cells. These fragments are presented on the surface of antigen-presenting cells (APCs) via major histocompatibility complex (MHC) molecules. This recognition process allows TCR-T cells to target a wider range of antigens within cancer cells.
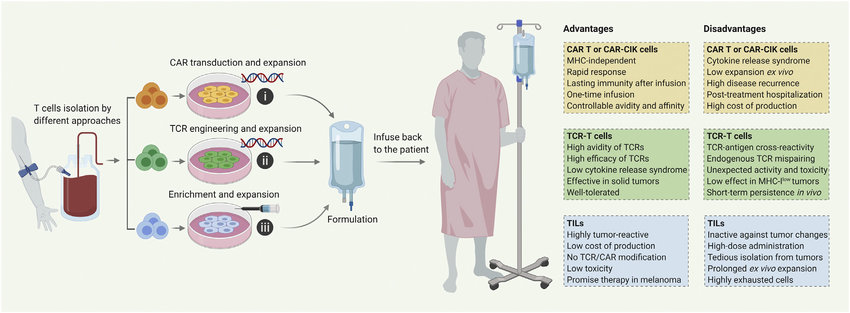
The development of TCR-T cell therapy involves isolating a patient's T cells, introducing a gene encoding a specific TCR that recognizes the targeted TAA, and then reintroducing the modified T cells back into the patient. These engineered T cells can then recognize and eliminate cancer cells expressing the targeted TAA.
Advantages of TCR-T Cell Therapy
- Broader targeting: TCRs can recognize a wider variety of antigens compared to CARs, potentially leading to more precise therapy and reduced side effects.
- Targeting internal antigens: TCR-T cells can target mutations within cancer cells, offering the possibility of eliminating tumors that lack surface antigens.
- Potential for memory response: TCR-T cells may develop a memory response against the targeted antigen, leading to long-term tumor control.
Challenges and Considerations
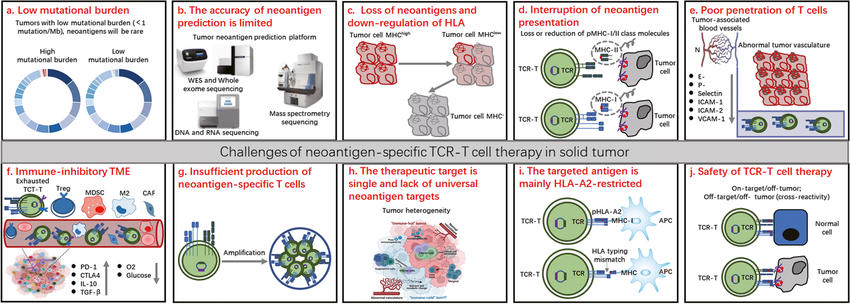
- MHC restriction: TCR recognition relies on MHC presentation, which may limit therapy to patients with compatible MHC alleles.
- Identifying ideal TCRs: Isolating and engineering highly effective and specific TCRs remains a challenge.
- Manufacturing complexity: The manufacturing process for TCR-T cells can be complex and time-consuming, potentially hindering widespread use.
The Future of TCR-T Cell Therapy
Researchers are actively working to address the limitations of TCR-T cell therapy. These efforts include:
- Developing universal TCRs: Engineering TCRs that function independently of MHC restriction to broaden applicability.
- In silico TCR design: Utilizing computational tools to predict and design high-affinity, tumor-specific TCRs.
- Next-generation manufacturing techniques: Streamlining manufacturing processes to improve efficiency and affordability.
Clinical trials exploring TCR-T cell therapy for various cancers are ongoing and demonstrating promising results. As research progresses in TCR identification, manufacturing processes, and overcoming MHC restrictions, TCR-T cell therapy holds immense potential as a targeted and potentially curative approach in cancer treatment.
The development of TCR-T cell therapy relies on robust research and development efforts, including the use of high-quality reagents for cell engineering and analysis. Companies like Maxanim are playing a crucial role in this progress by providing these essential reagents.
Learn more About TCR & T Cell in This Video:
TCR-T Cell Therapy: A Promising Approach in Cancer Treatment