Introduction
Cancer immunotherapy harnesses the body's own immune system to combat tumors. Neoantigen vaccines represent a burgeoning class of personalized immunotherapies demonstrating promise in clinical trials.
What are Neoantigens?
Neoantigens are mutated proteins unique to cancer cells. These mutations arise from errors during DNA replication or exposure to carcinogens. Since healthy cells lack neoantigens, the immune system can, in theory, recognize and target them, leading to tumor eradication.
The Rationale Behind Neoantigen Vaccines
Traditional cancer vaccines often target shared tumor antigens present in both cancerous and healthy cells. This approach can lead to autoimmune reactions. Neoantigen vaccines, conversely, focus on patient-specific mutations, minimizing the risk of autoimmunity while offering the potential for a highly targeted attack on cancer cells.
Development of Neoantigen Vaccines
The development of neoantigen vaccines hinges on two key steps:
- Tumor Neoantigen Identification: Next-generation sequencing technologies are used to identify tumor-specific mutations from a patient's tumor biopsy. These mutations are then translated into candidate neoantigens.
- Vaccine Design and Delivery: Candidate neoantigens are prioritized based on their predicted immunogenicity (ability to induce an immune response). The selected neoantigens are incorporated into a vaccine platform, such as dendritic cell vaccines, mRNA vaccines, or viral vectors, for delivery into the patient.
Efficacy of Neoantigen Vaccines
Clinical trials are investigating the efficacy of neoantigen vaccines in various cancers. Early results are encouraging. For instance, a recent study using an mRNA-based neoantigen vaccine (autogene cevumeran) in combination with immune checkpoint blockade and chemotherapy showed promising results in patients with pancreatic cancer.
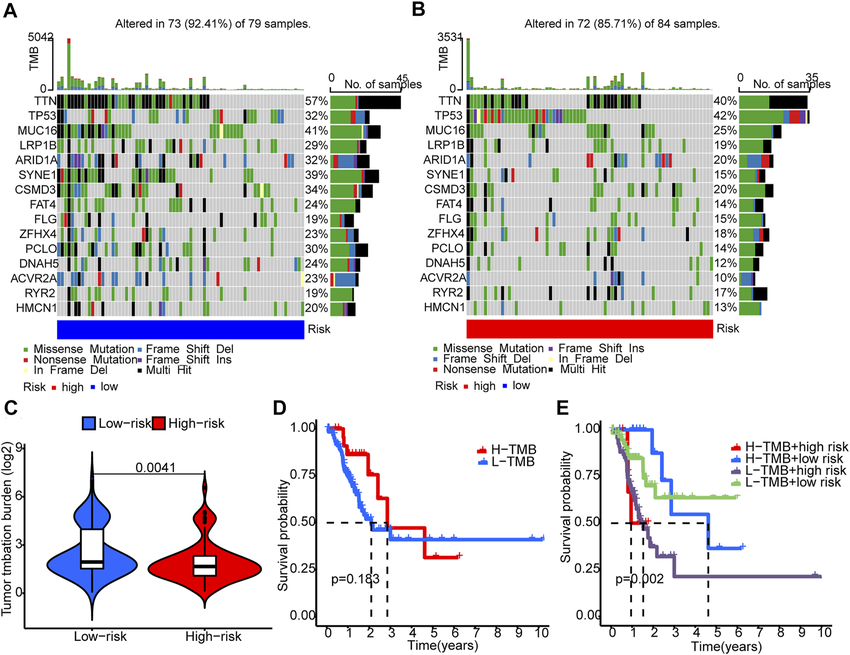
Tumor Mutational Burden (TMB) and Neoantigen Vaccines
TMB refers to the total number of mutations present in the cancer genome. Tumors with a high TMB are hypothesized to harbor more neoantigens, potentially making them more susceptible to immune attack by neoantigen vaccines. While research is ongoing, a high TMB might serve as a biomarker to identify patients likely to benefit from neoantigen vaccine therapy.
Ongoing Clinical Trials with Neoantigen Vaccines
The promise of neoantigen vaccines has spurred a multitude of clinical trials across various cancer types. These trials are evaluating the safety and efficacy of neoantigen vaccines, often in combination with other immunotherapies. Some ongoing trials are investigating neoantigen vaccines for melanoma, glioblastoma, and non-small cell lung cancer
Challenges and Future Directions
Despite the promise, neoantigen vaccines face challenges:
- Manufacturing Complexity: The personalized nature of these vaccines necessitates intricate manufacturing processes for each patient.
- Cost-Effectiveness: The current manufacturing methods can be expensive, hindering widespread adoption.
- Tumor Heterogeneity: Tumors can harbor a multitude of neoantigens, making it difficult to select the most immunogenic targets.
Ongoing research is focused on overcoming these challenges. Advancements in automation and streamlined manufacturing processes hold promise for cost reduction. Additionally, researchers are exploring strategies to target a broader spectrum of neoantigens within a tumor to enhance therapeutic efficacy.
Conclusion
Neoantigen vaccines represent a significant advancement in personalized cancer immunotherapy. While challenges persist, ongoing research is paving the way for their broader application in clinical oncology.
Future Considerations
Further exploration of TMB as a biomarker for neoantigen vaccine efficacy and continued monitoring of ongoing clinical trials will be crucial for the advancement of this promising therapeutic approach.
Explore the potential of neoantigen vaccines in this video.
Neoantigen Vaccines: A Personalized Approach to Cancer Immunotherapy