Introduction
Many neurodegenerative diseases, like Alzheimer's and Parkinson's, involve proteins folding incorrectly and clumping together. These clumps, called protein aggregates, damage cells in the brain and lead to disease. Therapies that prevent or break apart these aggregates are a promising approach for treating these diseases.
Challenges
Developing such therapies is difficult. First, we need to target only the harmful clumps, not the less harmful protein forms. Targeting the wrong structures could cause problems. Second, delivering treatments to the brain is challenging because of a barrier that protects the brain from foreign substances. Finally, protein aggregates form and change over time, so treatments need to target them at different stages.
Therapeutic Approaches
Scientists are exploring several approaches:
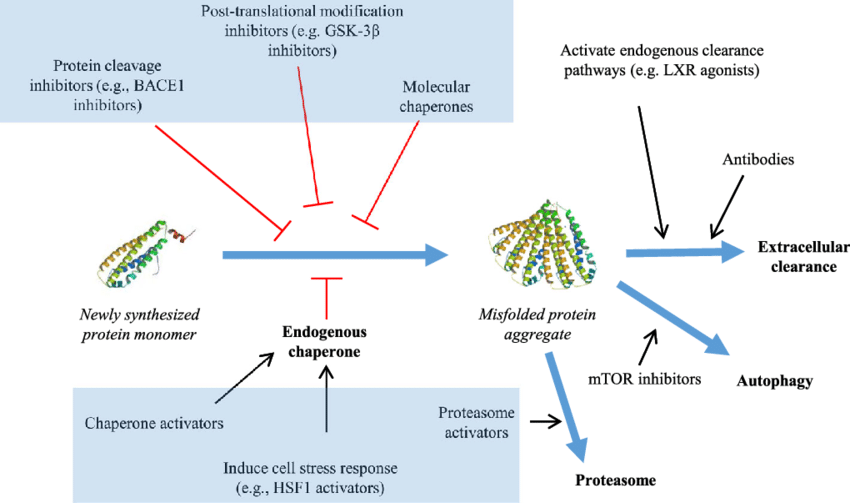
- Small molecules: These drugs bind to specific shapes of the misfolded protein, stopping them from clumping or breaking apart existing clumps. Traditional methods involve large-scale testing, while newer techniques use computer modeling to identify potential drugs.
- Molecular chaperones: These are natural proteins that help other proteins fold correctly and prevent clumping. Therapies aim to either boost the activity of existing chaperones or introduce new ones to promote proper folding.
- Antibodies: These are immune system proteins that can be engineered to recognize and attach to specific parts of protein aggregates. This approach is very specific and can target different stages of clumping.
- Protein degradation: Cells have natural ways to break down proteins, including the ubiquitin-proteasome system and autophagy. Therapies aim to improve these processes to clear out protein aggregates.
Researchers in the field of protein degradation, particularly those focusing on ubiquitination, are utilizing Nova Lifetech's plasmid range, provided by Maxanime (Gentaur group). For more details, please visit our website (click here).
- Gene therapy: This approach aims to fix the genetic mutations that cause protein misfolding in the first place. While promising for preventing disease, gene therapy faces safety and delivery challenges.
Future Directions
Research in this area is rapidly advancing. Combining different approaches, like small molecules with chaperone therapy, might be more effective. Additionally, developing ways to track protein aggregates inside living patients is crucial for testing therapies.
Conclusion
Targeting protein aggregates is a promising strategy for treating neurodegenerative diseases. Overcoming challenges like targeting the right structures, delivering treatments, and addressing different aggregate forms is key to developing effective therapies. Continued research has the potential to significantly improve the lives of patients with these diseases.
Learn more about the mechanisms of Alzheimer's disease by exploring the brain in this video:
![Molecular mechanisms of neurodegenerative diseases: Role of protein aggregation and neuronal network dysfunction. Protein aggregates deposited in brain subregions are a common characteristic of neurodegenerative diseases [180]. Extracellular and intracellular protein aggregates are commonly observed. The intracellular protein aggregation consists of (a) tau, (b) α-synuclein, (c) huntingtin protein, (d) SOD1 and (e) self-harm to neurons [181,182]. Each of these proteins are actively involved with cellular processes that play key roles in affecting microtubule and synaptic function [183]. However, amyloid-β, α-synuclein, and tau are also part of extracellular protein aggregates and stimulate astrocyte and oligodendrocyte responses. These occur with immunocytes to affect neuronal function and vitality [184]. Astrocytes, microglia and oligodendrocyte cytokines and ROS generate a spectrum of immune cell responses that leads to BBB, neural and glial damage [184,200]. Schematic illustration concept was adopted from [201]. Molecular mechanisms of neurodegenerative diseases: Role of protein aggregation and neuronal network dysfunction. Protein aggregates deposited in brain subregions are a common characteristic of neurodegenerative diseases [180]. Extracellular and intracellular protein aggregates are commonly observed. The intracellular protein aggregation consists of (a) tau, (b) α-synuclein, (c) huntingtin protein, (d) SOD1 and (e) self-harm to neurons [181,182]. Each of these proteins are actively involved with cellular processes that play key roles in affecting microtubule and synaptic function [183]. However, amyloid-β, α-synuclein, and tau are also part of extracellular protein aggregates and stimulate astrocyte and oligodendrocyte responses. These occur with immunocytes to affect neuronal function and vitality [184]. Astrocytes, microglia and oligodendrocyte cytokines and ROS generate a spectrum of immune cell responses that leads to BBB, neural and glial damage [184,200]. Schematic illustration concept was adopted from [201].](/web/image/42008-8a7a60c5/image.png?access_token=ea123fd0-93b4-4844-8bfe-10894c270ab0)
Targeting Protein Aggregates for Neurodegenerative Diseases